Search Result
About Us
Highlights
Blog
Highlights
Antigen tests are used in the diagnosis of respiratory pathogens and are immunoassays that detect the presence of a specific viral antigen, which implies current viral infection.
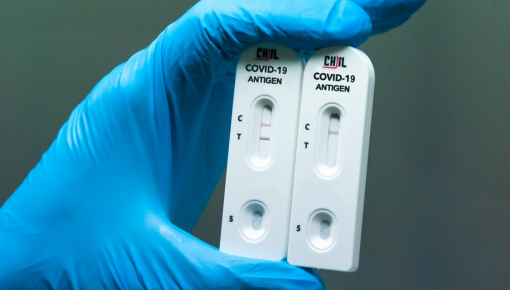
CHIL COVID-19 Antigen Rapid Test is designed to detect the presence of viral proteins (antigens) expressed by the COVID-19 virus in a sample from the respiratory tract of a person and is gives results in a few minutes whether someone is infectious. Approved by clinical specimens and authorities, this product is intended exclusively for professional use in the laboratory and at the point-of-care.
.

CHIL COVID-19 Antigen Rapid designed and created by CHIL's R&D team, approved by approved specimens. Based on independent laboratory and international authorities' report has 93.55% Specificity and a 100% accuracy.In this immune chromatography test, the sample will be under the capillary action to move forward along the test cassette. If the sample contains SARS-CoV-2 antigens, these antigens will be with colloidal gold-labeled coronavirus monoclonal antibody. This immune complex will be membrane fixed by coronavirus monoclonal antibody capture, form the purple line which reveals a positive result. If the line does not show any color, the negative result will be displayed. The test cassette also contains a quality control line C, which shall appear in purple regardless of whether there is a detection line. (Click here to see the Instruction for use manual)
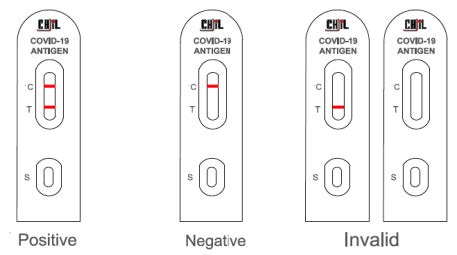
CE Certified CHIL COVID-19 Antigen Rapid Tests are currently authorized to be performed on nasopharyngeal or nasal specimens placed directly into the assay’s extraction buffer.
Nasopharyngeal Sampling Nasal Sampling
Get in contact with our sales team by clicking here to receive more information!
.
.
.
.
.
References
-https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html