CHIL's COVID-19 Response
Search Result
About Us
Highlights
Blog
Highlights
As the COVID-19 outbreak continues its spread globally, our thoughts are with the medical workers and people affected. As CHIL, we are eager to do our part during the pandemic by both ensuring our staff’s safety and by supporting health professionals, governments, and private businesses with our rapid tests. We acknowledge that in order to successfully guide this critical situation properly the public and private sectors should work together. As a medical device company, we are aware of our important role during this pandemic and working non-stop to do our part.
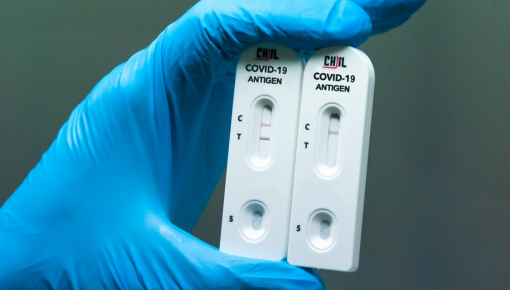
As a medical device manufacturer CHIL’s integral role emerges in responding to the COVID-19 pandemic. Taking quick and reliable actions, the fast development of diagnostic tests leads that cases are detected and quarantined rapidly.
At the beginning of the pandemic, CHIL launched serology blood tests to detect the specific antibodies produced towards SARS-CoV-2 and quickly began shipping its highly sensitive antibody tests to existing costumers after receiving relevant authorizations. CHIL’s COVID-19 IgG/IgM Rapid Test can detect IgG and IgM antibodies simultaneously within the same cassette. Laboratory studies are not decelerated and the performance studies are in full spate.

Testing is the key point globally to overcome the pandemic. It's crucial of course to help treat, isolate, or hospitalize people who are infected. Testing also is important in the bigger public health picture on mitigation efforts, helping investigators characterize the prevalence, spread, and contagiousness of the disease. Accurate and sensitive testing along with strong logistics; we are determined to play one of the most important roles during this outbreak.

Starting from the beginning of the pandemic, CHIL sped up its production, trained, and hired additional manufacturing employees to keep up with the increasing demand. Currently, operating along with 100 countries worldwide with its rapid tests.