Search Result
About Us
Highlights
Blog
Highlights
COVID-19 spread has been a significant part of our lives since December 2020, reported having originated in China’s Wuhan city and was declared as a pandemic by the World Health Organization (WHO) on 11 March 2020 as Italy, Iran, South Korea, and Japan reported surging numbers of cases. According to WHO, as of September 20, the global death toll surpassed 957,000 amid more than 30.8 million cases. Over 21 million people have recovered from the disease worldwide, according to the data collected by the Johns Hopkins University in the United States.
The SARS-CoV-2 virus affects different people in completely different ways. Some of the infected people demonstrate mild to moderate illness and recover without hospitalization. However, in some people, COVID-19 causes more severe symptoms like high fever, severe cough, and shortness of breath, which often indicates pneumonia*. Some people infected show no symptoms at all, which makes them harder to locate and isolate from the population in order not to endanger the rest.
Diseases such as influenza and pneumonia have clinical findings similar to COVID-19. Patients suffering from other respiratory system diseases during the COVID-19 pandemic can be misdiagnosed with COVID-19 disease. Likewise, there are people who have had COVID-19 who attribute the condition to a disease such as the flu. The CHIL IgG / IgM rapid test is specific to the disease and is not affected by cross-reaction. In this way, it will be possible to examine whether the disease is COVID-19 or not.
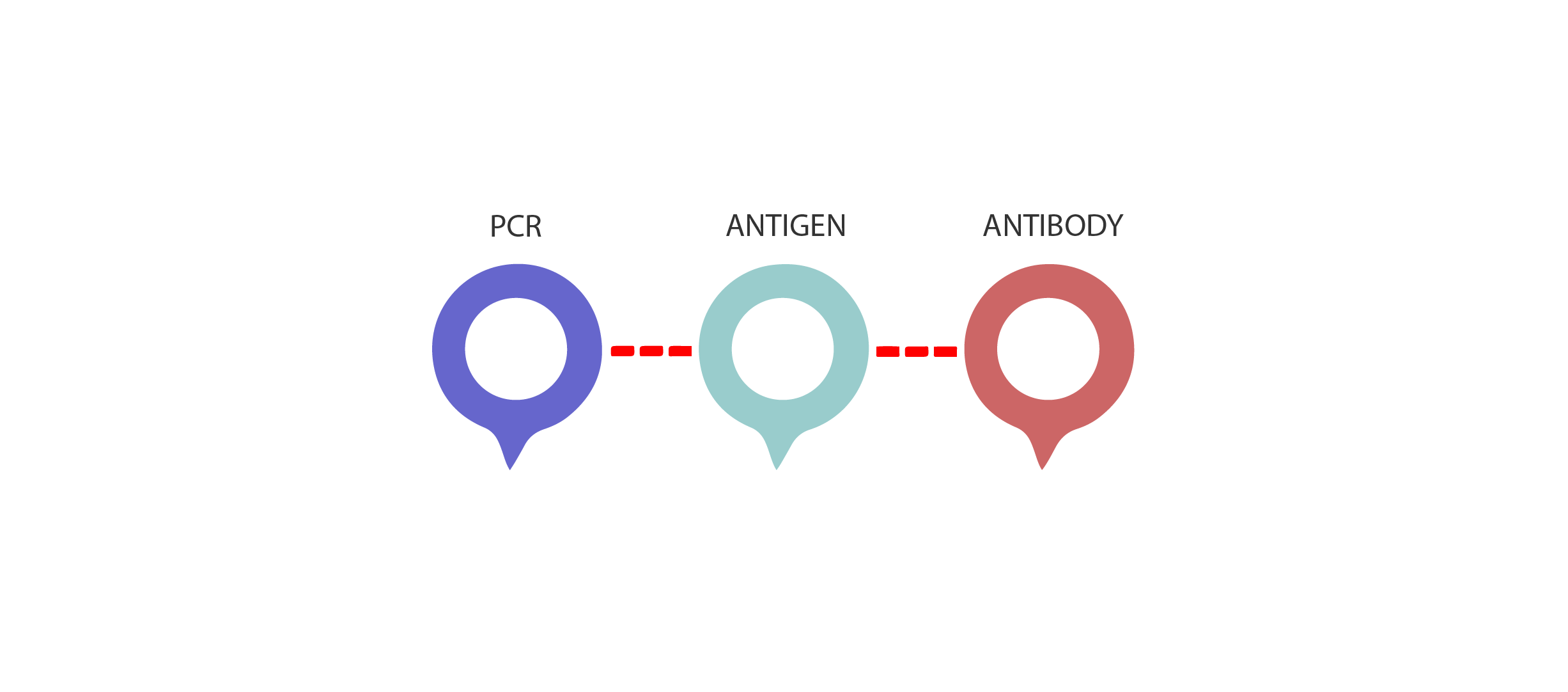
Currently, there are two kinds of tests are available for COVID-19 diagnosis; diagnostic tests and serology(antibody) tests. Diagnostic tests determine whether you have an active infection while antibody testing can tell if you had a past infection. Despite the universal recognition of PCR-based assays as a gold standard for molecular detection of viral diseases by government and medical councils, these methods require highly skilled personnel and sophisticated equipment, which makes them impractical in the setting of developing countries with limited resources. Because of PCR's inability to detect past infection and lack of highly skilled personnel along with the expensive equipment, the value of serological testing emerges.

Serology tests are tests that can detect the presence of antibodies quantitatively or qualitatively. The most used serology methods in the world are ELISA and lateral flow-based rapid tests. While the operator must be an expert in order to perform ELISA tests, rapid test methods are tests that can be applied in a wide range of environments and do not require individual expertise. The result interpretation is based on a very simple line logic so that it can understand whether it has been infected before. The interpretation process sheds light on the COVID-19 disease and allows the patient to comment on whether or not they have had an infection. The results of serology tests may be preliminary for diagnostic tests.
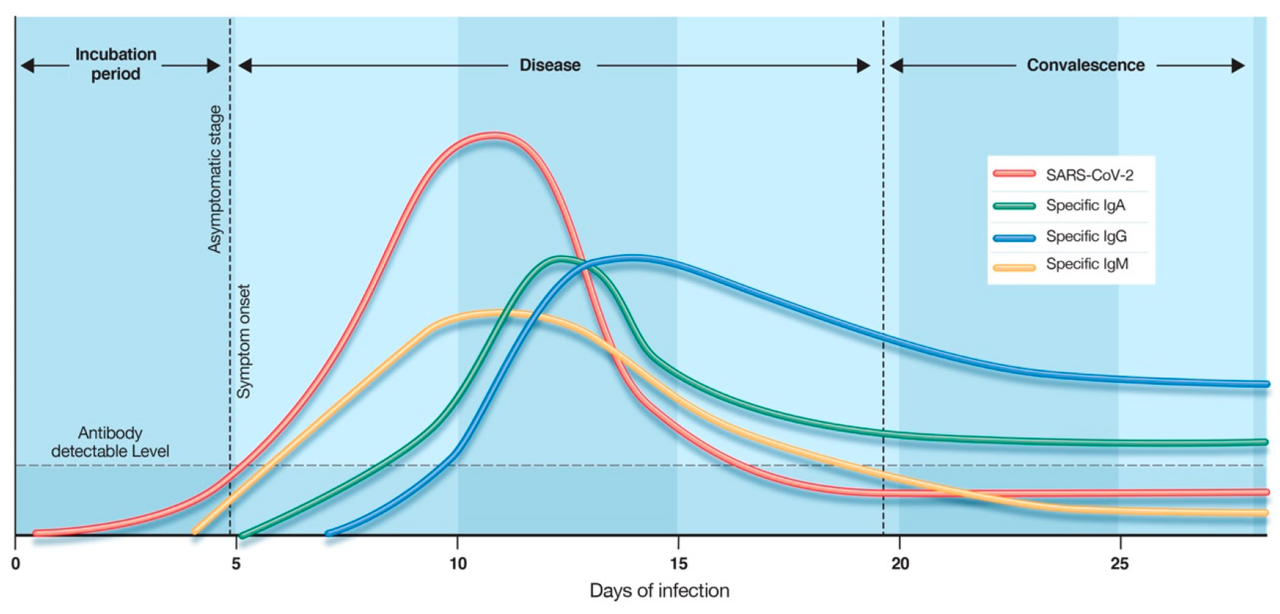
CHIL COVID-19 IgG/IgM Rapid Test is a serological rapid test that is performed by the blood looking for specific antibodies(IgG/IgM) created by your immune system towards SARS-CoV-2. Still, the infected individual does not begin producing antibodies quickly. CHIL COVID-19 IgG/IgM Rapid Test is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. It can take between seven days to three weeks for a serological antibody test to give positive.
Ghaffari, A., Meurant, R., & Ardakani, A. (2020). COVID-19 serological tests: how well do they actually perform? In Diagnostics (Vol. 10, Issue 7). https://doi.org/10.3390/diagnostics10070453
IgM antibody starts to be produced in the first week of the disease. IgG begins to be produced after the 7th day of the disease for long-term immunity. The positivity of these two antibodies gives useful information about the stages of the disease, immunity, and whether the person is infected. The CHIL IgG / IgM rapid test plays an important role in the fight against COVID-19, with an accuracy rate of over 98.9%, reliable results, and ease of use.